 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free! Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
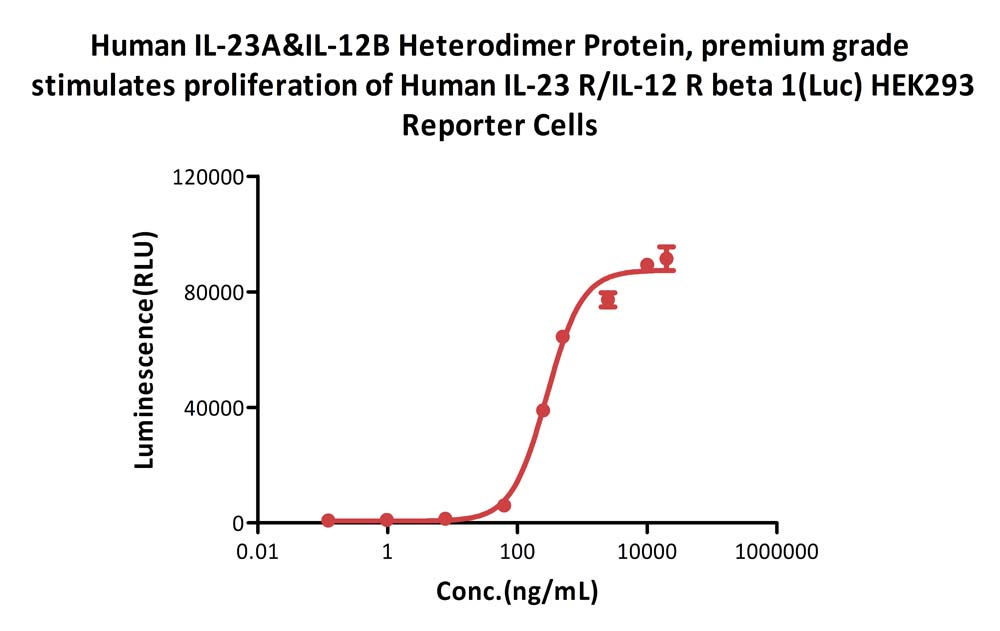
Human IL-23A&IL-12B Heterodimer Protein, premium grade (Cat. No. ILB-H5219) stimulates proliferation of Human IL-23 R/IL-12 R beta 1(Luc) HEK293 Reporter Cell. The specific activity of Human IL-23A&IL-12B Heterodimer Protein, premium grade is > 1.80 X 10^3 U/mg (QC tested).

Captured Biotinylated Human IL-23A&IL-12B Heterodimer Protein, His,Avitag&Tag Free (Cat. No. ILB-H82W6) on Biotin CAP - Series S sensor Chip can bind Human IL-23 R, His Tag (Cat. No. ILR-H52H4) affinity constant of 4.77 nM as determined in a SPR assay (Biacore T200) (Routinely tested).
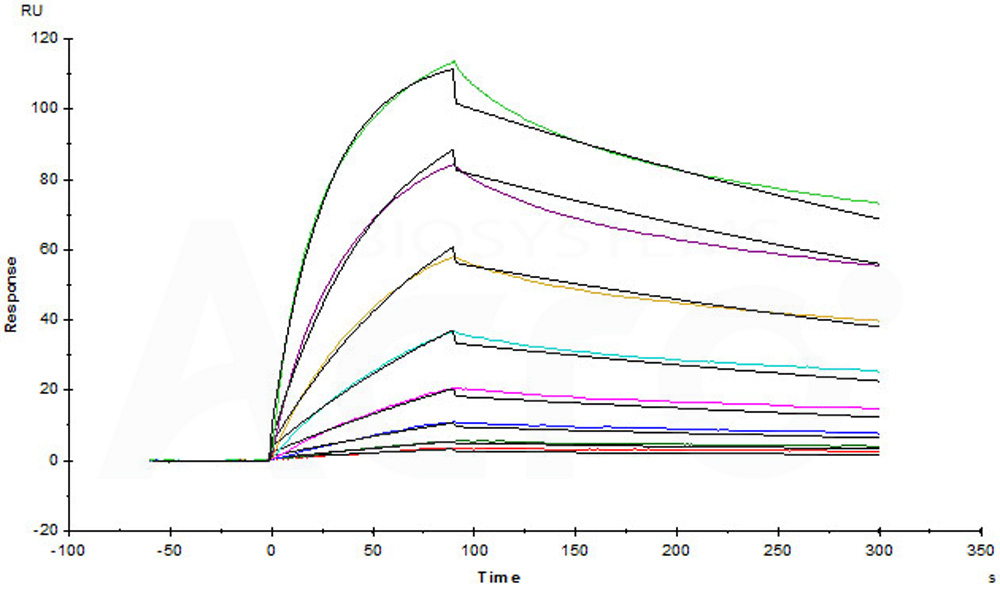
Captured Human IL-23 R, Fc Tag (Cat. No. ILR-H5254) on CM5 chip via anti-human IgG Fc antibodies surface can bind Human IL-23A&IL-12B Heterodimer Protein, His Tag&Tag Free (Cat. No. ILB-H52W5) with an affinity constant of 5.36 nM as determined in a SPR assay (Biacore T200) (Routinely tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Ustekinumab | CNTO-1275; C-340 | Approved | Johnson & Johnson Innovative Medicine | Stelara, 喜达诺 | United States | Plaque psoriasis | Janssen Biotech Inc | 2009-09-25 | Arthritis, Juvenile; Crohn Disease; Dermatitis, Atopic; Plaque psoriasis; Hidradenitis Suppurativa; Uveitis; Common Variable Immunodeficiency; Colitis, Ulcerative; Arthritis, Psoriatic; Takayasu Arteritis; Psoriasis; Lupus Erythematosus, Systemic; Liver Cirrhosis, Biliary; Multiple Sclerosis; Sjogren's Syndrome; Pouchitis; Ichthyosis; Inflammatory Bowel Diseases; Type I Leukocyte Adhesion Defect; Dermatomyositis; Non-radiographic axial spondyloarthritis; Diabetes Mellitus, Type 1; Polymyositis | Details |
| Ustekinumab biosimilar (Alvotech) | AVT04; AVT-04 | Approved | Alvotech hf | Jamteki, Uzpruvo, SELARSDI™ | Japan | Psoriasis | Fuji Pharma Co Ltd | 2023-09-25 | Psoriasis; Arthritis, Psoriatic; Plaque psoriasis; Crohn Disease | Details |
| Ustekinumab biosimilar(Amgen) | ABP-654 | Approved | Amgen Inc | Wezlana, WEZLANA | United States | Crohn Disease; Plaque psoriasis; Arthritis, Psoriatic; Colitis, Ulcerative | Amgen Inc | 2023-10-31 | Psoriasis; Arthritis, Psoriatic; Colitis, Ulcerative; Crohn Disease; Plaque psoriasis | Details |
| Ustekinumab | CNTO-1275; C-340 | Approved | Johnson & Johnson Innovative Medicine | Stelara, 喜达诺 | United States | Plaque psoriasis | Janssen Biotech Inc | 2009-09-25 | Arthritis, Juvenile; Crohn Disease; Dermatitis, Atopic; Plaque psoriasis; Hidradenitis Suppurativa; Uveitis; Common Variable Immunodeficiency; Colitis, Ulcerative; Arthritis, Psoriatic; Takayasu Arteritis; Psoriasis; Lupus Erythematosus, Systemic; Liver Cirrhosis, Biliary; Multiple Sclerosis; Sjogren's Syndrome; Pouchitis; Ichthyosis; Inflammatory Bowel Diseases; Type I Leukocyte Adhesion Defect; Dermatomyositis; Non-radiographic axial spondyloarthritis; Diabetes Mellitus, Type 1; Polymyositis | Details |
| Ustekinumab biosimilar (Alvotech) | AVT04; AVT-04 | Approved | Alvotech hf | Jamteki, Uzpruvo, SELARSDI™ | Japan | Psoriasis | Fuji Pharma Co Ltd | 2023-09-25 | Psoriasis; Arthritis, Psoriatic; Plaque psoriasis; Crohn Disease | Details |
| Ustekinumab biosimilar(Amgen) | ABP-654 | Approved | Amgen Inc | Wezlana, WEZLANA | United States | Crohn Disease; Plaque psoriasis; Arthritis, Psoriatic; Colitis, Ulcerative | Amgen Inc | 2023-10-31 | Psoriasis; Arthritis, Psoriatic; Colitis, Ulcerative; Crohn Disease; Plaque psoriasis | Details |
| Ustekinumab | CNTO-1275; C-340 | Approved | Johnson & Johnson Innovative Medicine | Stelara, 喜达诺 | United States | Plaque psoriasis | Janssen Biotech Inc | 2009-09-25 | Arthritis, Juvenile; Crohn Disease; Dermatitis, Atopic; Plaque psoriasis; Hidradenitis Suppurativa; Uveitis; Common Variable Immunodeficiency; Colitis, Ulcerative; Arthritis, Psoriatic; Takayasu Arteritis; Psoriasis; Lupus Erythematosus, Systemic; Liver Cirrhosis, Biliary; Multiple Sclerosis; Sjogren's Syndrome; Pouchitis; Ichthyosis; Inflammatory Bowel Diseases; Type I Leukocyte Adhesion Defect; Dermatomyositis; Non-radiographic axial spondyloarthritis; Diabetes Mellitus, Type 1; Polymyositis | Details |
| Ustekinumab biosimilar (Alvotech) | AVT04; AVT-04 | Approved | Alvotech hf | Jamteki, Uzpruvo, SELARSDI™ | Japan | Psoriasis | Fuji Pharma Co Ltd | 2023-09-25 | Psoriasis; Arthritis, Psoriatic; Plaque psoriasis; Crohn Disease | Details |
| Ustekinumab biosimilar(Amgen) | ABP-654 | Approved | Amgen Inc | Wezlana, WEZLANA | United States | Crohn Disease; Plaque psoriasis; Arthritis, Psoriatic; Colitis, Ulcerative | Amgen Inc | 2023-10-31 | Psoriasis; Arthritis, Psoriatic; Colitis, Ulcerative; Crohn Disease; Plaque psoriasis | Details |
| Ustekinumab | CNTO-1275; C-340 | Approved | Johnson & Johnson Innovative Medicine | Stelara, 喜达诺 | United States | Plaque psoriasis | Janssen Biotech Inc | 2009-09-25 | Arthritis, Juvenile; Crohn Disease; Dermatitis, Atopic; Plaque psoriasis; Hidradenitis Suppurativa; Uveitis; Common Variable Immunodeficiency; Colitis, Ulcerative; Arthritis, Psoriatic; Takayasu Arteritis; Psoriasis; Lupus Erythematosus, Systemic; Liver Cirrhosis, Biliary; Multiple Sclerosis; Sjogren's Syndrome; Pouchitis; Ichthyosis; Inflammatory Bowel Diseases; Type I Leukocyte Adhesion Defect; Dermatomyositis; Non-radiographic axial spondyloarthritis; Diabetes Mellitus, Type 1; Polymyositis | Details |
| Ustekinumab biosimilar (Alvotech) | AVT04; AVT-04 | Approved | Alvotech hf | Jamteki, Uzpruvo, SELARSDI™ | Japan | Psoriasis | Fuji Pharma Co Ltd | 2023-09-25 | Psoriasis; Arthritis, Psoriatic; Plaque psoriasis; Crohn Disease | Details |
| Ustekinumab biosimilar(Amgen) | ABP-654 | Approved | Amgen Inc | Wezlana, WEZLANA | United States | Crohn Disease; Plaque psoriasis; Arthritis, Psoriatic; Colitis, Ulcerative | Amgen Inc | 2023-10-31 | Psoriasis; Arthritis, Psoriatic; Colitis, Ulcerative; Crohn Disease; Plaque psoriasis | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Ustekinumab biosimilar(Formycon) | FYB-202 | Phase 3 Clinical | Psoriasis | Details | |
| Ustekinumab Biosimilar (qyuns) | QX-001-S; HDM-3001; QX-001S | Phase 3 Clinical | Qyuns Therapeutics Co Ltd | Psoriasis; Plaque psoriasis | Details |
| Ustekinumab Biosimilar(Celltrion) | CT-P43 | Phase 3 Clinical | Celltrion Inc | Psoriasis | Details |
| Ustekinumab biosimilar(Biocon) | Phase 3 Clinical | Biocon Biologics Ltd | Psoriasis | Details | |
| Ustekinumab biosimilar(CSPC Pharma) | SYSA-1902 | Phase 3 Clinical | Jushi Biopharmaceutical Co Ltd | Psoriasis; Plaque psoriasis | Details |
| Brazikumab | AMG-139; MEDI-2070; Anti-IL-23-MAb (Amgen) | Phase 2 Clinical | Amgen Inc | Inflammatory Bowel Diseases; Psoriasis; Colitis, Ulcerative; Crohn Disease | Details |
| anti-cancer mRNA therapeutics (Moderna Therapeutics) | mRNA-2752 | Phase 1 Clinical | Moderna Inc, Astrazeneca Plc | Solid tumours; Squamous Cell Carcinoma of Head and Neck; Triple Negative Breast Neoplasms; Neoplasms; Carcinoma, Transitional Cell; Lymphoma; Lymphoma, Non-Hodgkin; Carcinoma, Non-Small-Cell Lung; Melanoma | Details |
| LY-900021 | LY-900021 | Phase 1 Clinical | Halozyme Therapeutics Inc, Eli Lilly And Company | Details | |
| IL-23/CGRP bispecific antibody (Lilly) | Phase 1 Clinical | Eli Lilly And Company | Autoimmune Diseases | Details | |
| Ustekinumab Biosimilar (NeuClone/Serum Institute of India) | Phase 1 Clinical | Neuclone, Serum Institute Of India Ltd | Autoimmune Diseases | Details | |
| Ustekinumab biosimilar(Rani Therapeutics) | RT-111 | Phase 1 Clinical | Rani Therapeutics Llc | Psoriasis | Details |
| SOR-102 | SOR-102 | Phase 1 Clinical | Sorriso Pharmaceuticals Inc | Inflammatory Bowel Diseases; Colitis, Ulcerative | Details |
| Ustekinumab biosimilar (BioFactura) | BFI-751 | Phase 1 Clinical | BioFactura Australia Pty Ltd | Psoriasis | Details |
| Kagocel | Clinical | Nearmedic Plus Llc | Respiratory Tract Infections; Respirovirus Infections; Influenza, Human | Details | |
| Ustekinumab biosimilar(Formycon) | FYB-202 | Phase 3 Clinical | Psoriasis | Details | |
| Ustekinumab Biosimilar (qyuns) | QX-001-S; HDM-3001; QX-001S | Phase 3 Clinical | Qyuns Therapeutics Co Ltd | Psoriasis; Plaque psoriasis | Details |
| Ustekinumab Biosimilar(Celltrion) | CT-P43 | Phase 3 Clinical | Celltrion Inc | Psoriasis | Details |
| Ustekinumab biosimilar(Biocon) | Phase 3 Clinical | Biocon Biologics Ltd | Psoriasis | Details | |
| Ustekinumab biosimilar(CSPC Pharma) | SYSA-1902 | Phase 3 Clinical | Jushi Biopharmaceutical Co Ltd | Psoriasis; Plaque psoriasis | Details |
| Brazikumab | AMG-139; MEDI-2070; Anti-IL-23-MAb (Amgen) | Phase 2 Clinical | Amgen Inc | Inflammatory Bowel Diseases; Psoriasis; Colitis, Ulcerative; Crohn Disease | Details |
| anti-cancer mRNA therapeutics (Moderna Therapeutics) | mRNA-2752 | Phase 1 Clinical | Moderna Inc, Astrazeneca Plc | Solid tumours; Squamous Cell Carcinoma of Head and Neck; Triple Negative Breast Neoplasms; Neoplasms; Carcinoma, Transitional Cell; Lymphoma; Lymphoma, Non-Hodgkin; Carcinoma, Non-Small-Cell Lung; Melanoma | Details |
| LY-900021 | LY-900021 | Phase 1 Clinical | Halozyme Therapeutics Inc, Eli Lilly And Company | Details | |
| IL-23/CGRP bispecific antibody (Lilly) | Phase 1 Clinical | Eli Lilly And Company | Autoimmune Diseases | Details | |
| Ustekinumab Biosimilar (NeuClone/Serum Institute of India) | Phase 1 Clinical | Neuclone, Serum Institute Of India Ltd | Autoimmune Diseases | Details | |
| Ustekinumab biosimilar(Rani Therapeutics) | RT-111 | Phase 1 Clinical | Rani Therapeutics Llc | Psoriasis | Details |
| SOR-102 | SOR-102 | Phase 1 Clinical | Sorriso Pharmaceuticals Inc | Inflammatory Bowel Diseases; Colitis, Ulcerative | Details |
| Ustekinumab biosimilar (BioFactura) | BFI-751 | Phase 1 Clinical | BioFactura Australia Pty Ltd | Psoriasis | Details |
| Kagocel | Clinical | Nearmedic Plus Llc | Respiratory Tract Infections; Respirovirus Infections; Influenza, Human | Details | |
| Ustekinumab biosimilar(Formycon) | FYB-202 | Phase 3 Clinical | Psoriasis | Details | |
| Ustekinumab Biosimilar (qyuns) | QX-001-S; HDM-3001; QX-001S | Phase 3 Clinical | Qyuns Therapeutics Co Ltd | Psoriasis; Plaque psoriasis | Details |
| Ustekinumab Biosimilar(Celltrion) | CT-P43 | Phase 3 Clinical | Celltrion Inc | Psoriasis | Details |
| Ustekinumab biosimilar(Biocon) | Phase 3 Clinical | Biocon Biologics Ltd | Psoriasis | Details | |
| Ustekinumab biosimilar(CSPC Pharma) | SYSA-1902 | Phase 3 Clinical | Jushi Biopharmaceutical Co Ltd | Psoriasis; Plaque psoriasis | Details |
| Brazikumab | AMG-139; MEDI-2070; Anti-IL-23-MAb (Amgen) | Phase 2 Clinical | Amgen Inc | Inflammatory Bowel Diseases; Psoriasis; Colitis, Ulcerative; Crohn Disease | Details |
| anti-cancer mRNA therapeutics (Moderna Therapeutics) | mRNA-2752 | Phase 1 Clinical | Moderna Inc, Astrazeneca Plc | Solid tumours; Squamous Cell Carcinoma of Head and Neck; Triple Negative Breast Neoplasms; Neoplasms; Carcinoma, Transitional Cell; Lymphoma; Lymphoma, Non-Hodgkin; Carcinoma, Non-Small-Cell Lung; Melanoma | Details |
| LY-900021 | LY-900021 | Phase 1 Clinical | Halozyme Therapeutics Inc, Eli Lilly And Company | Details | |
| IL-23/CGRP bispecific antibody (Lilly) | Phase 1 Clinical | Eli Lilly And Company | Autoimmune Diseases | Details | |
| Ustekinumab Biosimilar (NeuClone/Serum Institute of India) | Phase 1 Clinical | Neuclone, Serum Institute Of India Ltd | Autoimmune Diseases | Details | |
| Ustekinumab biosimilar(Rani Therapeutics) | RT-111 | Phase 1 Clinical | Rani Therapeutics Llc | Psoriasis | Details |
| SOR-102 | SOR-102 | Phase 1 Clinical | Sorriso Pharmaceuticals Inc | Inflammatory Bowel Diseases; Colitis, Ulcerative | Details |
| Ustekinumab biosimilar (BioFactura) | BFI-751 | Phase 1 Clinical | BioFactura Australia Pty Ltd | Psoriasis | Details |
| Kagocel | Clinical | Nearmedic Plus Llc | Respiratory Tract Infections; Respirovirus Infections; Influenza, Human | Details | |
| Ustekinumab biosimilar(Formycon) | FYB-202 | Phase 3 Clinical | Psoriasis | Details | |
| Ustekinumab Biosimilar (qyuns) | QX-001-S; HDM-3001; QX-001S | Phase 3 Clinical | Qyuns Therapeutics Co Ltd | Psoriasis; Plaque psoriasis | Details |
| Ustekinumab Biosimilar(Celltrion) | CT-P43 | Phase 3 Clinical | Celltrion Inc | Psoriasis | Details |
| Ustekinumab biosimilar(Biocon) | Phase 3 Clinical | Biocon Biologics Ltd | Psoriasis | Details | |
| Ustekinumab biosimilar(CSPC Pharma) | SYSA-1902 | Phase 3 Clinical | Jushi Biopharmaceutical Co Ltd | Psoriasis; Plaque psoriasis | Details |
| Brazikumab | AMG-139; MEDI-2070; Anti-IL-23-MAb (Amgen) | Phase 2 Clinical | Amgen Inc | Inflammatory Bowel Diseases; Psoriasis; Colitis, Ulcerative; Crohn Disease | Details |
| anti-cancer mRNA therapeutics (Moderna Therapeutics) | mRNA-2752 | Phase 1 Clinical | Moderna Inc, Astrazeneca Plc | Solid tumours; Squamous Cell Carcinoma of Head and Neck; Triple Negative Breast Neoplasms; Neoplasms; Carcinoma, Transitional Cell; Lymphoma; Lymphoma, Non-Hodgkin; Carcinoma, Non-Small-Cell Lung; Melanoma | Details |
| LY-900021 | LY-900021 | Phase 1 Clinical | Halozyme Therapeutics Inc, Eli Lilly And Company | Details | |
| IL-23/CGRP bispecific antibody (Lilly) | Phase 1 Clinical | Eli Lilly And Company | Autoimmune Diseases | Details | |
| Ustekinumab Biosimilar (NeuClone/Serum Institute of India) | Phase 1 Clinical | Neuclone, Serum Institute Of India Ltd | Autoimmune Diseases | Details | |
| Ustekinumab biosimilar(Rani Therapeutics) | RT-111 | Phase 1 Clinical | Rani Therapeutics Llc | Psoriasis | Details |
| SOR-102 | SOR-102 | Phase 1 Clinical | Sorriso Pharmaceuticals Inc | Inflammatory Bowel Diseases; Colitis, Ulcerative | Details |
| Ustekinumab biosimilar (BioFactura) | BFI-751 | Phase 1 Clinical | BioFactura Australia Pty Ltd | Psoriasis | Details |
| Kagocel | Clinical | Nearmedic Plus Llc | Respiratory Tract Infections; Respirovirus Infections; Influenza, Human | Details |
This web search service is supported by Google Inc.
